Water is a Universal Solvent, but Why?
We cannot imagine life without water. All living things depend upon water in various degrees, and for a good reason. The reason is, water is a universal solvent. What does that mean, and why is water a universal solvent? Today we will set out to explore this.
Water is, of course, the most common chemical compound in the world. Hell, two-thirds of the planet’s surface is covered by it! And there are few things occurring in the natural world that do not get dissolved in water, to some varying degree. And that exactly is why we call water a universal solvent ‒ it dissolves mostly everything.

This is an extremely important matter in the cycle of nature. Water dissolves all the required salts and nutrients and other chemicals required to support life and carries them from one point to another. All life depends upon this phenomenon. For example, plants are built to suck water out of the ground and there is all the food dissolved in that water that the plant needs. Water is the largest part of the blood that circulates through all living things’ bodies, carrying nutrients to every cell and waste material out of the body as urine and sweat.
The definition of a universal solvent is difficult to give. Naturally, you’d think that means a substance that can dilute anything. But as you can further imagine, this is far from practical ‒ every solvent will have at least one (hundreds!) similar chemical with similar properties that will be inert in that solvent, not a solute. So we have to widen our definition of a universal solvent as the substance that can dissolve the most amount of chemicals.
Water is, by and far, the best solvent in the world. It can make solutions out of most compounds, save a few organic materials. The reason for that is that water is one of the best polarized compounds capable of breaking up the ionic bonds present in most compounds.
What does that mean? Let’s discuss.
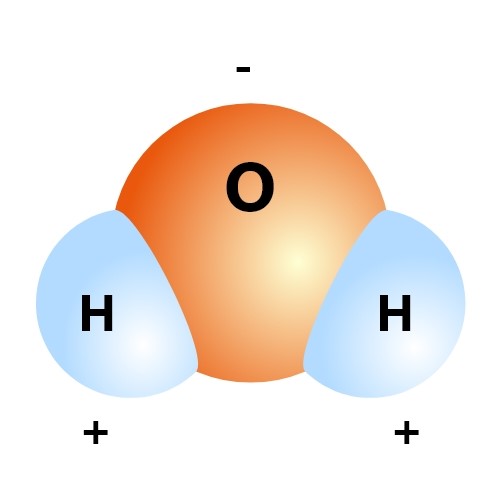
The proper chemical name of water is Dihydrogen Oxide, and the chemical symbol is H2O. In this compound, there are two hydrogen atoms and one oxygen atom bound by a covalent bond. This means that this compound should be perfectly balanced electrically. The oxygen atom, however, is somewhat greedy and pulls the shared electrons closer to itself. This results in a slightly negative charge on the oxygen’s side and an equal positive charge on the hydrogen atoms’ side.
Now, that is very attractive to chemicals that have bonds based on electrical charge; that is, ionic-bonded compounds. Let’s take the example of Sodium Chloride, the common salt. The copper part of each molecule of NaCl carries a strong positive charge, while the Chloride is the anion with an equal negative charge. Normally, they are connected to each other in this opposites-attract sort of situation and form crystals
When you drop some of that crystallized powder into some water, silent mayhem starts. The water molecules are polarized with one side negative and the other positive, and they normally live together in harmony aligned by that. But as soon as you drop something with a bit more charge ‒ like this NaCl in question ‒ the molecules break their communal hydrogen bond and rush the ionic compound.
The H2O molecules crowd around the cations and anions present in the salt, pulling them apart. All the water molecules near the cation Na+ spin to face the negative oxygen side to face the cation, while the water molecules near the negative Cl- ions latch onto them by the positive hydrogen side.
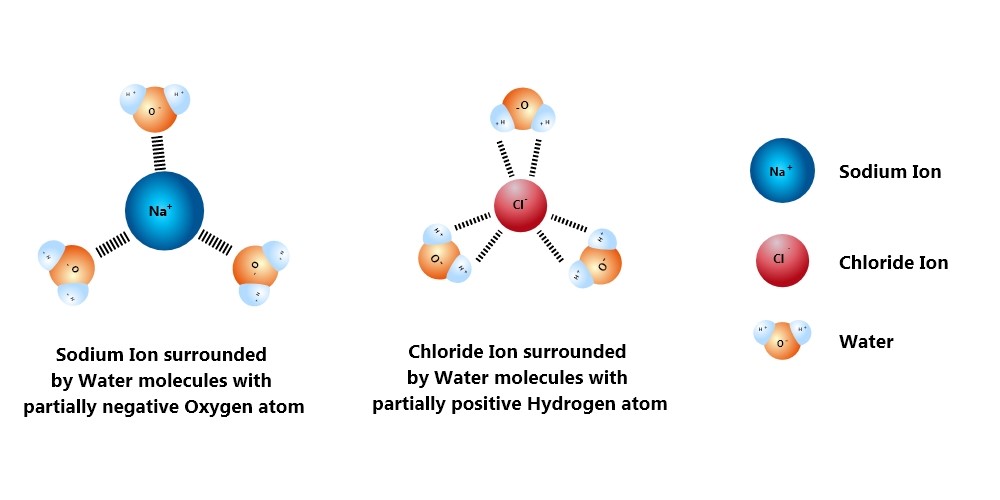
The pull into the crowd of the H2O molecules grows stronger as more and more of them attach themselves to the salt ions. The Sodium and the chloride ions are very strongly bonded, but there are just too many water molecules around them hungrily clamping on. Like ants dismembering a dead cockroach, the anion and the cation are pulled apart mercilessly.
Once the salt is broken down into its basic cation and anion, these clusters of ions with water molecules drift around since they are perfectly balanced now. So the salt becomes homogeneously spread all over the water body.
This ability of H2O to break compounds into ions is what makes it the best solvent all around. Obviously, this power is not the same for all chemicals ‒ there are some chemicals like salt and sugar that just love to get dissolved in water, and there are some carbonates, etc. that have great resistance against it. However, the dissolving power of water gets much more intense when it is a little bit acidic ‒ which is very easy to happen in nature. That is exactly what causes natural corrosion in structures .
Water is such a good solvent that it can dissolve not only solids but also other liquids (duh) and even gasses. Yes, you heard that right. How do you think fish breathe? They get the oxygen that is dissolved in the water. By just being in contact with air, much of it gets dissolved in water.
Frustratingly for scientists, this super-solvent capability of water is exactly why it is very hard to get it in completely pure form. Pure H2O is never found ‒ there is almost always something diluted in it, be it simple gases from the air. Truly, given pure enough water and enough time, it can even break down plastic which is supposedly impervious to water!

This leads us to comment on one thing. Since water cannot, apparently, live alone, the more you purify it the more it will become hungry for salts. This is why it is not a good idea to drink water that is too pure ‒ it will dilute whatever salts and minerals and nutrients it can find in your body and take it away while going out, making you weaker in the process.
That’s one of the biggest reasons why drinking water purifiers are intentionally made to never completely purify the water even when they can. Sure, they kill off all the germs in water (even that may not be a very good idea) but keep the salts in. Of course, water purifiers used in laboratories are different ‒ they produce much purer water that is highly dangerous to drink. You can also buy demineralized water from Labkafe which is pretty much free of any cations and anions.












Leave a Reply