We have already discussed why water is the best solvent all-around in a previous blog article; but what about the things that get dissolved in water? There are sugar and salts and various other stuff that get dissolved in water, but salts have the best solubility in water. Now, why should that be?
In chemistry labs you may have noticed that various chemical salts get dissolved in water so easily; you just have to shake the test tube with the salt and water to make an original solution . But at home, you may have seen that you have to work the sugar with a spoon a lot; even in hot water. Normal table salt gets dissolved much faster, doesn’t it?
There is a very good reason for that. But we have to delve deep into the matter of solubility to understand it.

The definition of solubility tells us that the solubility of a given substance is max how much of it can make a clear, transparent solution with a given solvent at a given temperature. Taking water as the solvent (it is, in most cases), we can see that inorganic salts are more soluble than anything else. The reason for that lies in the manner of how things get dissolved.
There are two ways something can be dissolved in water, which depends upon the kind of compound it is. As you may know, there are two kinds of chemical bonds that form compounds: (a) Covalent bonds and (b) ionic bonds. Most inorganic salts are formed by ionic bonds and most other stuff are covalent compounds.
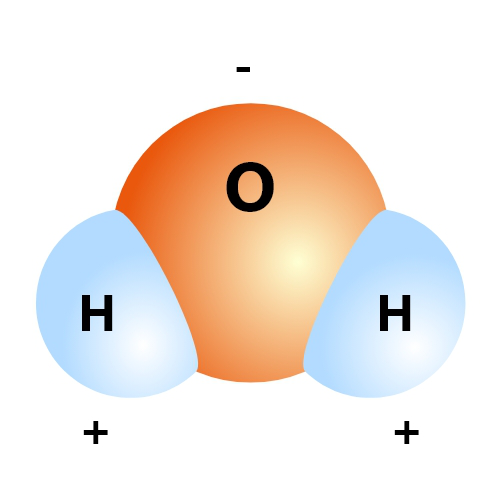
Water itself is a covalent compound. That means, each hydrogen atom in H2O shares an electron with the oxygen atom, which shares two of its own with the hydrogens. Similar stuff happens with other covalent compounds and they form generally very stable molecules.
When a covalent solid compound like sugar touches water, there is no chemical interaction. There are a bunch of sugar molecules huddled together at one side, and touching them are a bunch of water molecules held much loosely together. The water molecules simply seep inside the gaps between the sugar molecules. The process is entirely physical and there are no chemical reactions or interactions going on here. This phenomenon can be quite slow, considering how well the covalent compound is held together.
What happens with ionic compounds ‒ is much more dramatic!
To begin with, there is no such thing as a molecule of an ionic compound. Surprised? Don’t be. The very nature of the ionic bond is that there is no actual bond between two ions ‒ they are just strongly attracted to each other. But unlike covalent compounds, there is no actual binding contract here.
For this reason, ionic compounds form lattices and crystals much better than other compounds. Take common table salt for example ‒ Sodium chloride. Each Na+ ion had sacrificed an electron to become positively charged; each Cl- ion stole an electron from somewhere and got negatively charged. Opposites attract; and so all Na cations and Cl anions in the vicinity rush together and form a kind of structure where every sodium atom is surrounded by six chlorines and vice-versa. There is no single “NaCl” floating around as we may imagine. They are a joint family or nothing.
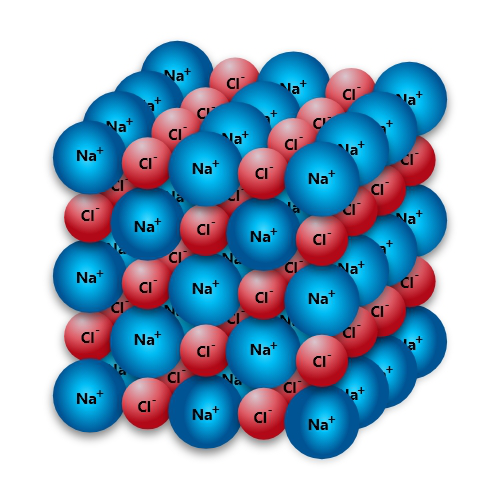
This is a pretty strong structure as solid things go. There are few things in the world that may entice a sodium or a chloride ion to leave the group and do something, unless the thing itself is much more charged than them. Like some strong reagent.
We’ll let you in on a little secret. Water is such a charged thing.
How can that be, you must be asking. Wasn’t water supposed to be a covalent compound? How can it be charged? Water molecules exist, don’t they?
Well, let us explain. There is one special thing about water, that makes it such a curious chemical. The oxygen atom in every dihydrogen oxide (water) molecule is a greedy person and attracts all the electrons around it towards itself; even those in the covalent bonds with the hydrogen atoms are pulled closer to the oxygen atom.
Naturally, with all the electrons crowding together around the oxygen atom, that side of the molecule becomes somewhat negatively charged. Whereas the hydrogen sides get positively charged in contrast. This makes water a ‘polarized’ compound ‒ one of the very few.
This is the very property of water that makes it so efficient at pulling apart most ionic compounds. Let’s consider our example again. When the sodium chloride structure comes in contact with polarized water, a silent mayhem begins.
The positive side of the water molecules latches onto the negatively charged chloride ions, and the negative oxide side sticks to the positive sodium ions. This by itself isn’t enough to break the attraction bond between Na and Cl.
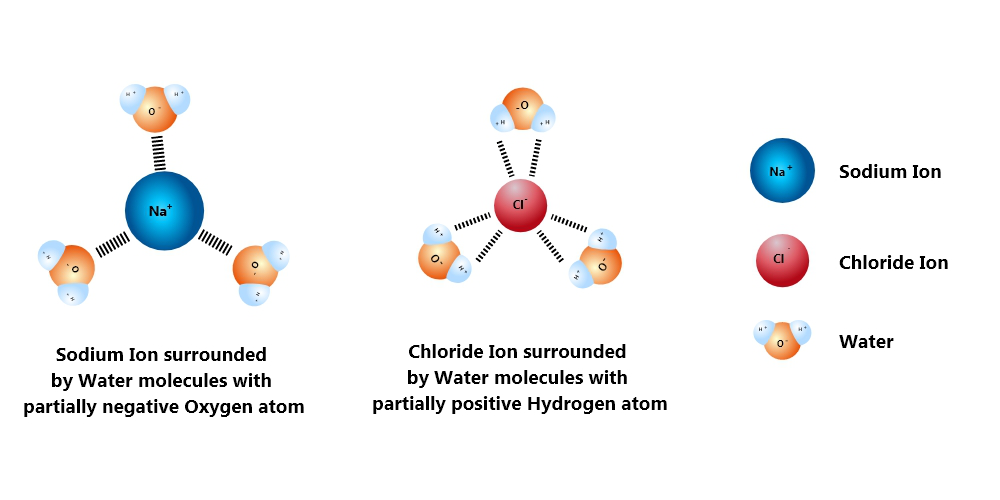
But there are so many water molecules crowding around each of those ions! Like a colony of ants killing a spider, lots of water molecules crowd around each atom, each contributing its own little force. At one point, the combined strength of the water molecules becomes greater than the force between the Na and Cl ions. Like ants pulling apart a dead cockroach, they are mercilessly torn apart.
Each anion and cation of a salt falling into water float around, packed within a bunch of water molecules latched onto them by small electrical charges. Effectively separated, the salt gets quickly spread all around the water body, dissolving better than anything else.
Almost all chemical inorganic salts available in the salt analysis practical of class 10-12 behave like this; that’s why these salts are so easily soluble in water. Of course, there are a few exceptions. Here is a table of common salt solubility for your convenience.
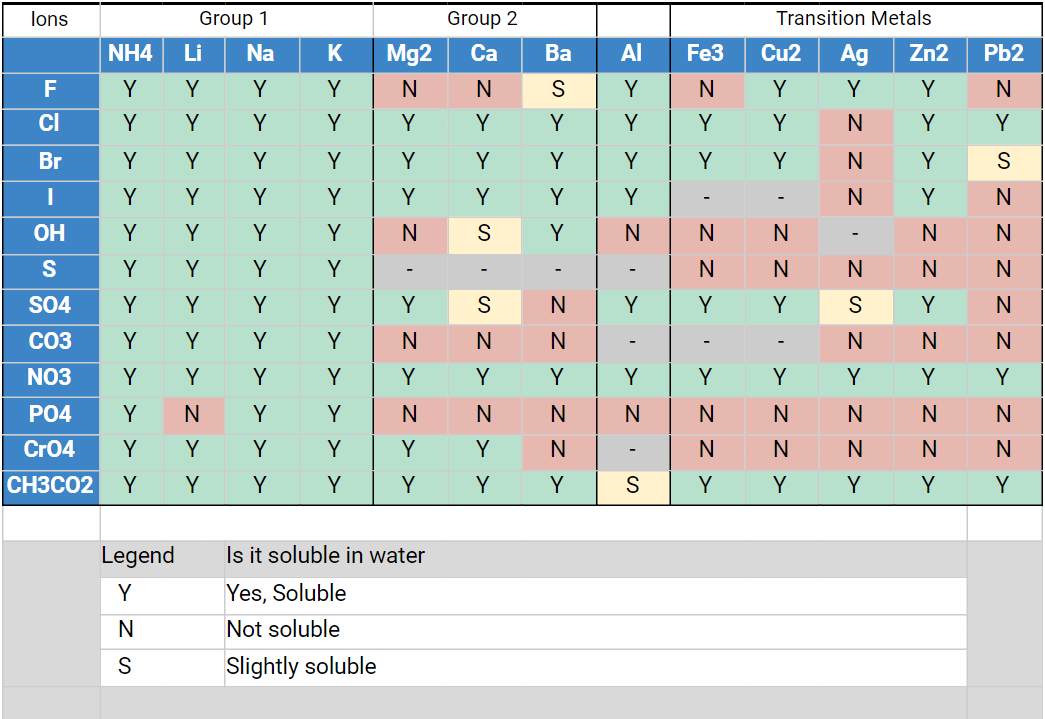
In the table above, the cations are arranged horizontally and the anions are arranged vertically. Thereby, you can combine the ions column-by-row and see clearly which salts will be soluble in water, which salts are insoluble in water (but they can be if encouraged by adding a little conc. HCl), and which are slightly soluble in water (meaning you will have to heat the water).
As you can see, the board is mostly green across lighter molecules. This lets you predict which types of salts will be more soluble in water and which will be less.
You can follow these rules of thumb to remember which salts will be soluble in water:
- All nitrates are soluble.
- All sodium, potassium and ammonium salts are soluble, except carbonates.
- If it’s a silver or lead salt, chances are it won’t be soluble.
These rules of thumb will let you make your way easier through the chemistry laboratory. Labkafe supplies most of those salts (soluble and insoluble) as part of the lab consumable package; but you can also buy them separately.








Leave a Reply