The Modern Periodic Table helps us predict the properties of elements based on their atomic number. Without this structure, identifying patterns in chemical behavior would be difficult and inconsistent. Earlier attempts to classify elements lacked a reliable basis, which made chemical trends harder to explain. Henry Moseley solved this in 1913 by organizing elements according to their atomic number—the number of protons in an atom’s nucleus. As a result, the table reveals repeating trends across rows (periods) and columns (groups), giving us a powerful tool to understand and predict the characteristics of elements.
Structure and Organization of the Modern Periodic Table
The Modern Periodic Table consists of 18 vertical columns called groups and 7 horizontal rows called periods. Elements in the same group have the same number of valence (outermost) electrons, which gives them similar chemical properties.
As we move down a group, the number of electron shells increases, but the number of valence electrons stays the same. In contrast, moving from left to right across a period increases the number of valence electrons by one with each step, since the atomic number also increases by one.
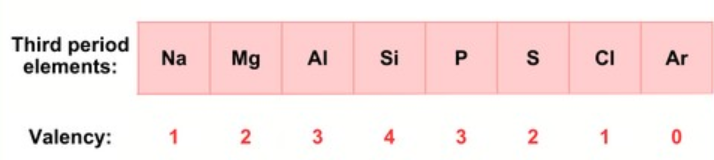
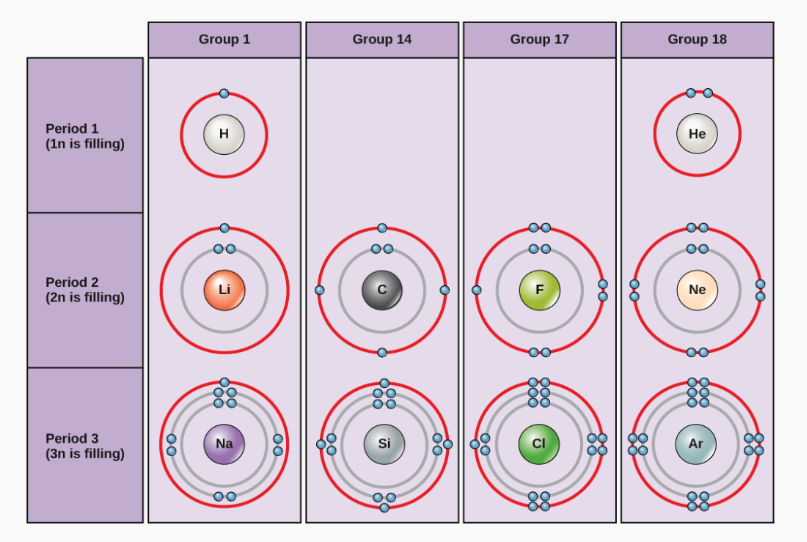
Take the third period as an example. Elements like Na, Mg, Al, Si, P, S, Cl, and Ar are arranged based on the filling of electrons into the K, L, and M shells. The maximum number of electrons each shell can hold is given by the formula 2n², where “n” represents the shell number from the nucleus.
For example, the K shell (n = 1) holds 2 electrons (2 × 1² = 2), so the first period has 2 elements. The L shell (n = 2) holds 8 electrons (2 × 2² = 8), giving the second period 8 elements. Although the M shell (n = 3) can hold up to 18 electrons (2 × 3² = 18), only 8 electrons are added in the third period due to limits on the number of electrons in the outermost shell.
Trends Observed in the Modern Periodic Table
Elements in the Modern Periodic Table follow systematic trends in their physical and chemical properties. Therefore, these trends help us predict how elements behave, bond, and react with other substances.
Valency
Valency is the number of valence electrons in an element’s outermost shell.
- Down a group: Valency remains the same because the number of valence electrons does not change.
- Across a period: Valency first increases from 1 to 4 as the number of valence electrons increases, then decreases from 4 to 0. For example, in Period 3, Na (valency 1) to Si (valency 4), then down to Ar (valency 0).
Atomic Size
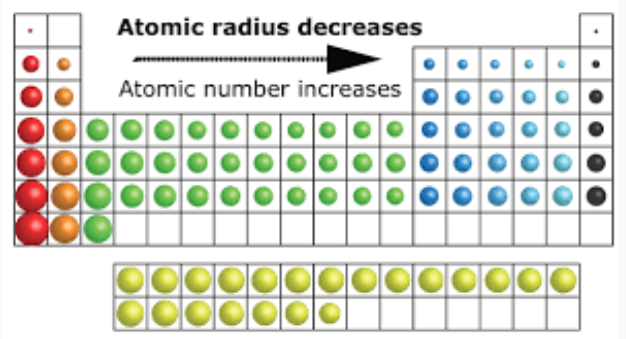
Atomic size is the distance between the nucleus and the outermost shell of an atom.
- Down a group: Atomic size increases. Consequently, new electron shells are added, increasing the distance between the nucleus and outermost electrons, despite the increasing nuclear charge.
- Across a period: Atomic size decreases. As the atomic number increases, the nuclear charge increases. This pulls the electrons closer to the nucleus, reducing atomic size.
Metallic and Non-metallic Character
Metallic character is the tendency of an atom to lose electrons and form positive ions (cations).
- Across a period: Metallic character decreases. Additionally, elements are less likely to lose electrons due to increasing nuclear attraction.
- Down a group: Metallic character increases. Valence shell electrons leave the atom easily.
Non-metallic character is the tendency to gain electrons to form negative ions (anions).
- Across a period: Non-metallic character increases.
- Down a group: Non-metallic character decreases.
Classification of Elements
- Metals are found on the left side of the table (e.g., Na, Mg). They are typically shiny, conductive, and form basic oxides.
- Non-metals are on the right side (e.g., S, Cl). They are poor conductors and usually form acidic oxides.
- Metalloids lie along a zig-zag line separating metals and non-metals. They have intermediate properties and include elements like B, Si, Ge, As, Sb, Te, and Po.
Effective Nuclear Charge and Reactivity
Across a period: The effective nuclear charge (net attraction experienced by outer electrons) increases. Therefore, this makes atoms hold onto their electrons more tightly, reducing their tendency to lose electrons.
Down a group: Increase in nuclear charge takes place. Overall attraction reduces due to added shells. Valence shell electrons leave the atom easily.
Reactivity trend:
- In metals: Reactivity increases down a group and decreases across a period.
- In non-metals: Reactivity decreases down a group and increases across a period.
Nature of Oxides
The type of oxide formed by an element depends on its position in the table.
- Metalloids may form amphoteric oxides that can act as both acids and bases. Example: Al₂O₃.
- Metallic oxides (left side) are generally basic. Example: Na₂O.
- Non-metallic oxides (right side) are generally acidic. Example: SO₂.
How to Memorize the Modern Periodic Table?
The best way to memorize the periodic table chart is through visualization. Then, use interesting mnemonics to aid memorization or consider purchasing a model like this. Then, our experts will guide you every step of the way!
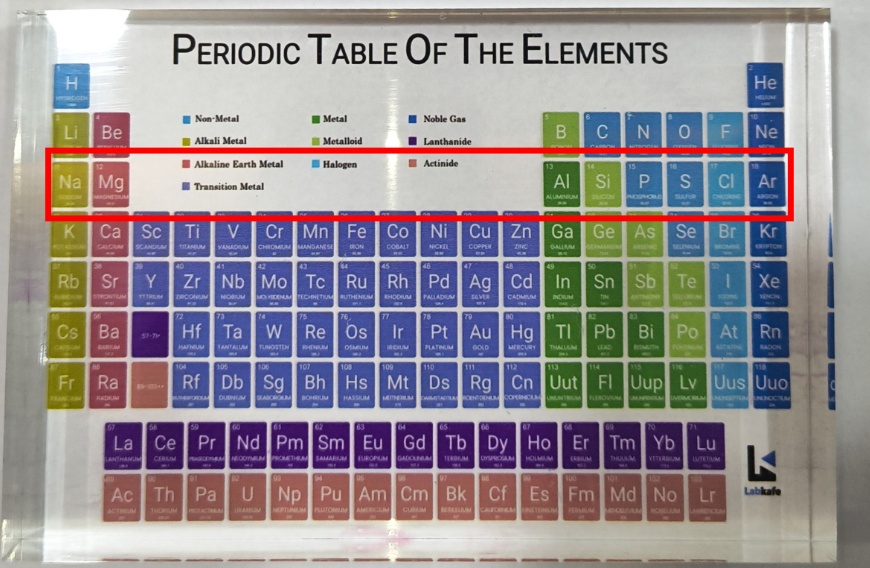
Keep it on your table and glance at it during your free time. Then, the attractive colors and tactile sensation of the model will help you retain the names of elements easily.





Leave a Reply