Table of Contents
- Who needs the laboratory hood?
- Which experiments need to be performed inside a laboratory hood?
- More Actions
- Frequently Asked Questions (FAQs)
Have you ever felt the strange smell of ammonia vapors or the stinging sensation of HCl fumes? Bad news—not only is the smell disgusting, but the vapors can also harm the lining of your nose and damage your lungs and respiratory system. Seeing this problem, chemists developed the laboratory hood, or fume hood, in the early 20th century. Since then, it has been the gold standard in laboratories that work with chemical substances releasing toxic fumes, vapors, or particles that can harm the user.
Who needs the laboratory hood?
Schools, colleges, research institutes, and industries that work with chemicals that are volatile, have low boiling points, and produce toxic vapors and fumes need to install laboratory or fume hoods. These filter and purify the air, emitting it outside or recirculating it into the lab. They absorb air from inside and around the fume hood when switched on. Even when the laboratory hood sash is closed, it can still pull in and purify air.
Which experiments need to be performed inside a laboratory hood?
Schools, colleges, research institutes and industries have separate categories of experiments that need a laboratory hood.

Laboratory Hoods for School Experiments
Schools normally follow a syllabi such that lab experiments are relatively safe. However, some experiments need to be performed inside a fume hood, especially since young children and relatively untrained students are involved.
Neutralization reactions
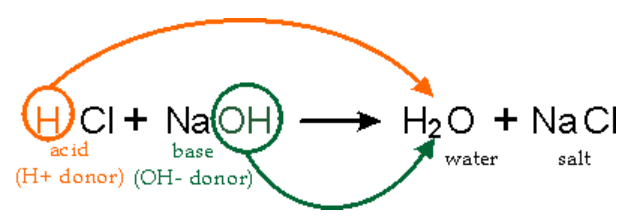
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
Dangers: HCl (aq) often emits acidic fumes that are harsh on the respiratory epithelium of the nose and lungs. Hence, laboratory hoods can be used to minimize the amount of HCl vapour inhaled by students during chemistry practicals.
Displacement reactions
Zn (s) + 2HCl (aq) → ZnCl₂ (aq) + H₂ (g)
Dangers: The issue of HCl vapors persists here. The H₂ gas that is emitted is flammable and cannot be allowed to accumulate inside the chemistry lab.
Combustion reactions
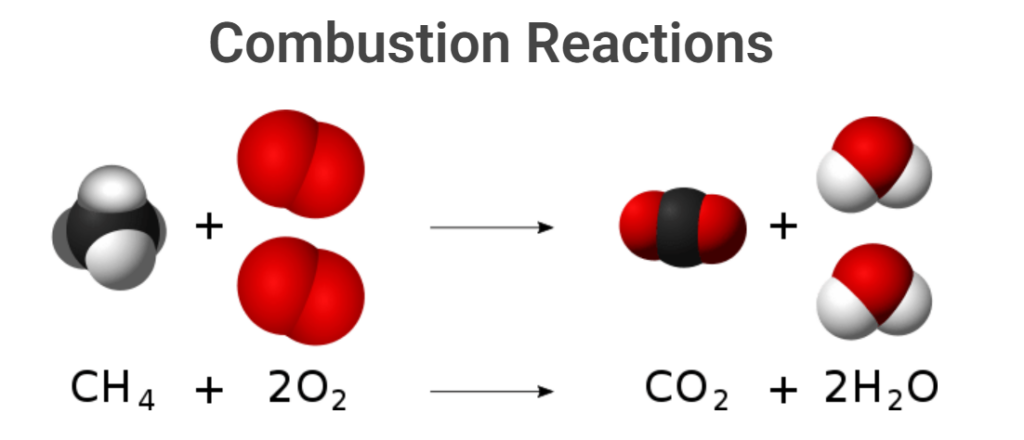
CH₄ (g) + 2O₂ (g) → CO₂ (g) + 2H₂O (g)
Dangers: Methane is highly flammable. This reaction should be conducted with complete precautions, such as a controlled supply of methane before combustion, proper dispersal, and ventilation in case of leakage. Regular checks are necessary to ensure that accidental leaks do not occur when no one is watching.
Additionally, the CO₂ formed is toxic if it accumulates inside the lab. It displaces oxygen and makes breathing difficult. When multiple students perform the experiment, a large amount of CO₂ will accumulate inside the lab, causing suffocation. Hence, it must be performed inside a laboratory hood.
Laboratory Hoods for Research Institutes
Research institutes deal with reactions that are generally not demonstrated at the school level. Some of these reactions can be dangerous, others emit toxic or flammable fumes necessitating fume hoods.
Oxidation of Organic Compounds
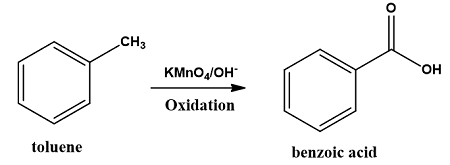
C₆H₅CH₃ (toluene) + KMnO₄(HCl) → C₆H₅COOH (benzoic acid)
Dangers: Toluene is highly flammable, irritant to the skin and mildly toxic when inhaled. Hydrochloric Acid is corrosive and releases HCl gas that is also corrosive. Hence, laboratory hoods can benefit scholars working in these settings.
Grignard Reactions
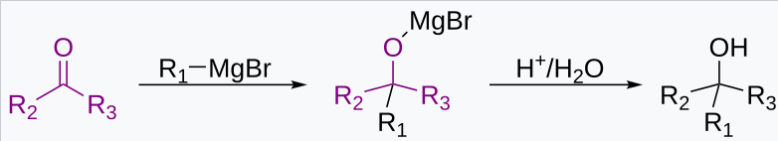
RMgX + R’COR” → R’R”RCOMgX
R’R”RCOMgX + H₂O → R’R”RCOH + Mg(OH)X
Dangers: Grignard reagents are dissolved in highly flammable ethers. The alkanes released are sometimes flammable. Hence the fume hood serves to remove alkane vapors from the lab. This reduces the chance of fire hazards.
Diazotization Reactions
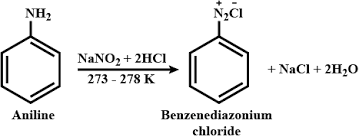
ArNH₂ + NaNO₂ + HCl → ArN₂⁺Cl⁻ + 2H₂O
Dangers: Certain conditions can produce nitrogen oxides (NOₓ), which are harmful when inhaled, as well as hydrochloric acid (HCl) vapors. These can be dissipated and safely vented using a laboratory hood.
Laboratory Hoods for Industrial and R&D Laboratories
Industrial labs usually test reactions in the R&D lab before scaling up to large-scale setups. This process is dangerous because, many times, the exact reaction dynamics and outcomes may not be known beforehand. Theoretically, some reactions can be performed more safely in fume hoods.
Chlorination Reactions
C₂H₄ + Cl₂ → C₂H₄Cl₂
Dangers: Chlorine must be handled carefully. The vapour is extremely toxic and can cause respiratory damage. Laboratory hoods can remove the vapours from the air, if excess chlorine is dissipated inside the lab.
Nitration of Aromatic Compounds
C₆H₆ + HNO₃ → C₆H₅NO₂ + H₂O
Dangers: Nitric and hydrochloric acids are used in this reaction. Both emit acidic pungent vapours that must be safely handled inside the laboratory hood.
Polymerization of Acrylates
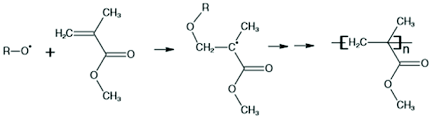
n CH₂=CHCOOCH₃ → [CH₂-CH(COOCH₃)]ₙ
Dangers: Methyl methacrylate vapors are heavier than air. They can settle down and cause difficulty in breathing due to irritation in the respiratory system. They can also cause irritation in the eyes and on the skin. It is highly flammable when mixed with air, with a closed cup flash point of 2°C.
More Actions
Science Lab Equipment Supplies
Learn how to maintain laboratory hoods
Frequently Asked Questions (FAQs)
Which chemical is dangerous in the laboratory?
A list of dangerous chemicals found in the lab according to MIT are:
- Arsenic trioxide
- Chlorine
- Hydrogen cyanide
- Nitrous oxide
- Phosgene
- Potassium cyanide (analytical reagent and purified)
- Sodium arsenate (analytical reagent)
- Sodium cyanide (analytical reagent)
For experiments with chlorine or nitrous oxide, a laboratory hood must be used.
What is a dangerous science experiment in school?
A dangerous science experiment is one where safety hazards, such as toxic substances, flammable materials, or high temperatures, can lead to harm if proper precautions are not followed. One example of a risky science demonstration is the Rainbow Flame Demonstration, where different salts are mixed with ethanol or methanol to produce colored flames.
Methanol, commonly used in such demonstrations, is highly toxic by inhalation and skin contact. It also releases heavy vapors that can travel long distances and has a dangerously low flash point. When working with methanol, you may use a laboratory hood.












Leave a Reply