
The various types of chemistry lab flasks available today can be confusing to choose from. However, specific experiments and laboratory procedures require specific types of flasks. Choosing the wrong one can lead to wasted resources, unsafe conditions, or poor experimental results.
This guide will walk you through the different types of chemistry lab flasks, their materials, uses, and how to choose the right one for your work. Whether you’re preparing for a titration, heating solutions, or running a vacuum filtration, this information will prove very useful once you’re at your laboratory bench.
Types of Chemistry Lab Flasks and Their Uses
Materials Used in Chemistry Lab Flasks
Glass Chemistry Lab Flasks
Most laboratory flasks are made from borosilicate glass, because it resists thermal shock and harsh chemical reactions. Other options include quartz glass or fused silica, which are suitable for operations requiring high temperatures, UV transparency, or extreme chemical resistance. Glass chemistry lab flasks offer transparency, chemical resistance, and temperature tolerance essential for safe lab work.
Plastic Chemistry Lab Flasks
Plastic flasks are made from materials like fluoropolymer resins, polyamides, rubber, elastomers, and other proprietary polymers. These flasks are useful when break resistance and flexibility are needed. For instance, EPDM (ethylene propylene diene monomer) rubber flasks resist ultraviolet (UV) light well but are unsuitable for petroleum-based applications because they break down in the presence of fuels.
Resistant plastic chemistry lab flasks include:
- PTFE (Polytetrafluoroethylene): Chemically inert and can resist high temperatures.
- PFA (Perfluoroalkoxy alkane): Offers protection from contamination, chemical resistance, and flexibility.
- FEP (Fluorinated ethylene propylene): They exhibit outstanding resistance to virtually all chemicals, retain their flexible, shatterproof properties down to liquid nitrogen temperatures, and are usable to temperatures over 200°C. You can clean them rigorously in boiling nitric acid. It is also guaranteed leak proof.
6 Common Types of Chemistry Laboratory Flasks
Erlenmeyer Flask

Invented by German chemist Emil Erlenmeyer, this flask has a flat bottom, broad base, and narrow cylindrical neck. It’s ideal for storing and heating liquid reagents. Its tapered shape makes it suitable for swirling liquids without spillage, which is especially useful during titrations. Additionally, the narrow neck reduces evaporation and prevents airborne contaminants from entering. However, you cannot make accurate volume measurements with it.
Volumetric Flask

You can use this chemistry lab flask for analytical chemistry when preparing precise solution concentrations. It has a rounded or flat base and a long narrow neck with etched volume markings. You can perform highly accurate volume measurements of liquids and mix standard solutions, such as aqua regia (a combination of hydrochloric acid and nitric acid).
Büchner Flask
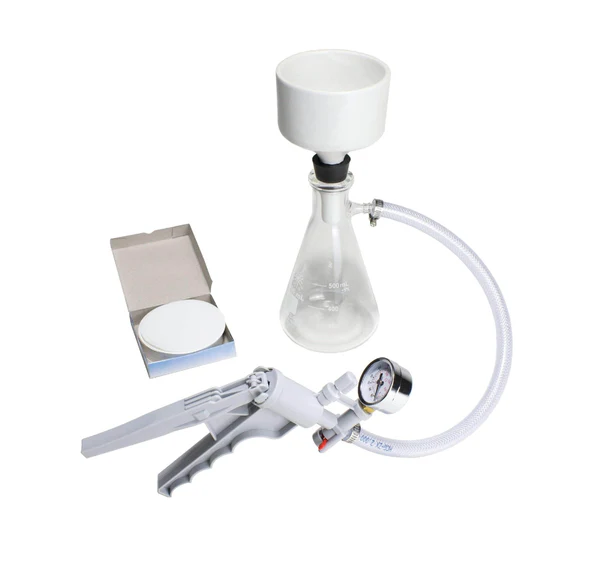
Also called a filter flask, vacuum flask, or side-arm flask, Ernst Büchner invented it. It resembles an Erlenmeyer flask but has thicker glass walls (to withstand high pressure) and a short neck. A sidearm allows connection to a vacuum pump for vacuum filtration using a Büchner funnel. This setup helps separate solids from liquids quickly and efficiently.
Round-Bottom Flask

Created by Otto Schott, the round-bottom flask features a spherical base and narrow neck. You need to support it with a clamp or ring stand, as it cannot stand upright on its own. This design ensures even heat distribution, making it ideal for boiling or heating. The narrow neck limits evaporation. Some versions include multiple necks for chemical additions or inserting gas lines to inject noble gases like argon to facilitate inert atmosphere reactions.
Reagent Flask

These chemistry lab flasks store solid or liquid chemicals. You will also see ones made with amber or black glass to block light and prevent photochemical degradation of sensitive substances. They provide safe and stable storage for daily laboratory use.
Distillation Flask

This flask has a rounded bottom, long neck, and an angled sidearm. It’s used in distillation processes to separate liquid mixtures based on their boiling points. As the solution is heated, components vaporize at different temperatures, and the vapor exits through the sidearm for condensation and collection.
Specialized Laboratory Flasks
Kohlrausch Volumetric Flask

Used in the Kohlrausch method, a titration technique for determining reducing sugars. A sugar sample is reacted with a modified Fehling’s solution (copper (II) sulfate), then the leftover copper (II) ions are titrated using sodium thiosulfate. The difference gives the sugar content. You can use this flask for the Kohlrausch process.
Kjeldahl Flask

Used in the Kjeldahl method to determine organic nitrogen content in substances like food and soil. The sample is digested with sulfuric acid, converting the nitrogen to ammonium sulfate. Later, distillation and titration steps follow. This flask is shaped to allow safe digestion under high heat.
Iodine Flask

This flask is designed for iodometric titrations, since iodine is a volatile substance. It includes a stopper to prevent iodine loss through evaporation, ensuring accuracy. While it resembles an Erlenmeyer flask, it is typically more expensive due to its specialized sealing and chemical resistance.
Saybolt Flask

Used in Saybolt viscometers to determine the viscosity of petroleum products. The process involves heating the fluid in the flask to a specific temperature and allowing it to flow through a calibrated orifice. The time taken to flow a fixed volume is used to calculate viscosity in Saybolt Universal Seconds (SUS).
Mojonnier Flask

You can use this for fat extraction, especially in dairy testing. The process involves mixing the sample with ethyl ether and petroleum ether to dissolve fats. The flask’s shape facilitates separation and extraction of dry fat, which is measured after evaporation of solvents.
Le Chatelier Flask

This flask helps determine the density of hydraulic cement, fly ash, lime, or other fine powders. It has a long-graduated neck and a bulbous base. Add the powder to a known volume of liquid inside the flask. Note the rise in liquid level which helps calculate the substance’s density accurately, using the Archimedes’ Principle.
Schlenk Flask

Used for air-sensitive or moisture-sensitive reactions, it has two necks: one for adding chemicals and another for connecting to a Schlenk line, which supplies an inert gas like argon or nitrogen. This setup allows reactions to occur without exposure to air or moisture, protecting highly reactive chemicals.
Florentine Flask

You can use a Florentine flask for extraction of essential oils, particularly from plants. It works by separating water from oil based on their different densities during steam distillation. Collect the oil that floats above the water.
Pear-Shaped Flask

This flask has a rounded “V” shape, making it ideal for collecting solid residues after evaporation. It is easy to scrape solids from its inner surface, unlike flat or round-bottom flasks. It also allows for easier liquid extraction with a syringe due to its tapering base.
Winkler Oxygen Bottle

Used in the Winkler method to measure dissolved oxygen in water. The method involves adding reagents like manganese sulfate and potassium iodide to the water sample. The oxygen reacts with the reagents to form a precipitate, which is later acidified and titrated with sodium thiosulfate. The amount of titrant used determines the oxygen concentration, indicating water quality.
Frequently Asked Questions (FAQs)
What are the three types of volumetric flasks?
You can use volumetric flasks to prepare solutions with high precision.
Standard Volumetric Flask
- Shape: Round or flat bottom with a long, narrow neck marked with a single calibration line.
- Use: Preparing and diluting standard solutions with precise volume.
- Application: Common in analytical chemistry for tasks requiring accurate concentrations.
Kohlrausch Volumetric Flask
- Shape: Similar to a standard volumetric flask but used in a specific analytical method.
- Use: Supports the Kohlrausch method, a titration process used to determine reducing sugars.
- Application: Mainly in food chemistry to analyze sugar content in samples like juices and syrups.
Le Chatelier Flask
- Shape: Bulb at the base with a narrow, graduated neck.
- Use: Measures the density of powders like cement, lime, fly ash, and similar fine materials.
- Application: Commonly used in construction material testing labs for quality control.












Leave a Reply