Titration of Mohr’s Salt with KMnO4 explained in detail with complete theory and procedure for Board Exams in India.
Table of Contents
- Titration of Mohr’s Salt with KMnO4: Theory
- Titration of Mohr’s Salt with KMnO4: Apparatus
- Titration of Mohr’s Salt with KMnO4: Procedure
- Titration of Mohr’s Salt with KMnO4: Calculation
- Results and Discussion
- Precautions
- Frequently Asked Questions (FAQ)/ VIVA
Titration of Mohr’s Salt with KMnO4: Theory
Potassium permanganate is a strong oxidant in the presence of sulfuric acid. Mohr salt is a double salt forming a single crystalline structure having the formula FeSO4.(NH4)2SO4.6H2O. The chemical name for Mohr’s salt is ferrous ammonium sulfate.
In this titration Mohr salt acts as a reducing agent and potassium permanganate acts as an oxidizing agent. Acid Base Titration with Mohr’s Salt against KMnO4 is an example of redox reaction. In this redox reaction, ferrous ion from Mohr’s salt gets oxidized and pink colored of manganese present in potassium permanganate, which is in the +7-oxidation state gets reduced to colorless Mn2+ state.
The chemical reaction and the molecular chemical equation is given below.
Reduction half reaction –
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
Oxidation half reaction –
[2FeSO4(NH4)2SO4.6H2O + H2SO4 + [O] → Fe2(SO4)3 + 2(NH4)2SO4 + 13H2O] x 5
Overall reaction –
2KMnO4 + 10FeSO4(NH4)2SO4.6H2O+ 8H2SO4 → K2SO4+ 2MnSO4+ 5Fe2(SO4)3+ 10(NH4)2SO4+ 68H2O
Titration of Mohr’s Salt with KMnO4: Apparatus
We will need the following to perform Acid Base Titration with Mohr’s Salt Against KMnO4 as an example:
- Burette – Filled with KMnO₄ (Potassium Permanganate) solution
- Conical Flask – Contains 10 mL of Ferrous Ammonium Sulfate (Mohr’s salt) and sulfuric acid
- Pipette – To accurately measure and transfer Mohr’s salt solution
- Funnel – To fill the burette without spillage
- Retort Stand with Clamp – To hold the burette securely
- White Tile (optional) – To enhance visibility of the color change at the endpoint
- Distilled Water – For dilutions and rinsing apparatus
- Wash Bottle – For rinsing the conical flask

Burettes for Acid Base Titration Example Mohr’s Salt Against KMnO4 for Board Exam Practical by Labkafe
LABKAFE provides all necessary apparatus for Chemistry practical exams for CBSE, ICSE, and all State Boards, along with compatible lab furniture. Browse our compact Chemistry Lab Packages (CBSE, ISC and State Boards) which includes every piece of equipment needed for your specific board exam practical. For any queries or requirements, get in touch with our lab experts today!
Titration of Mohr’s Salt with KMnO4: Procedure
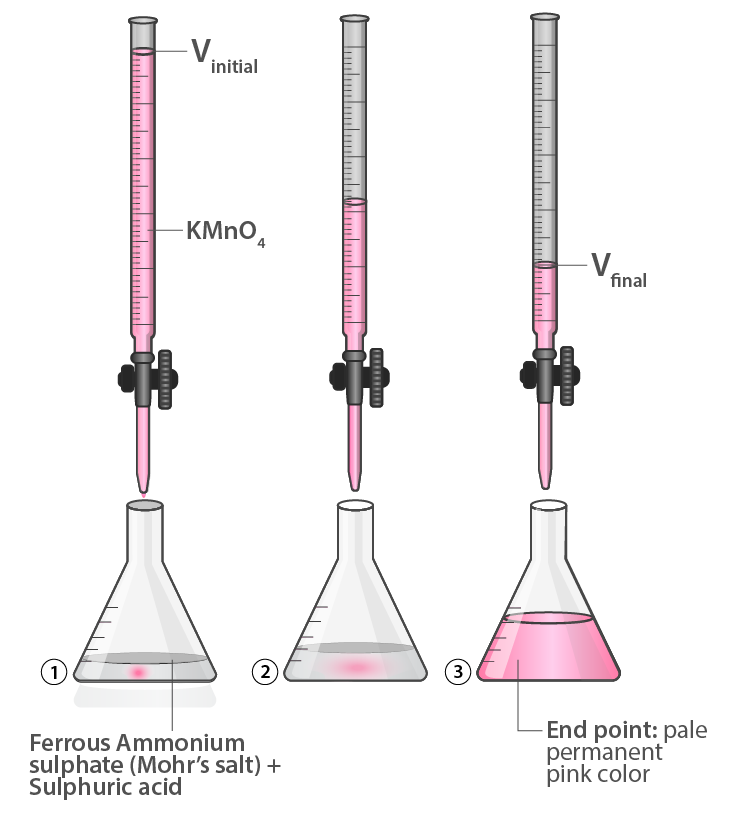
Setup for Acid Base Titration example with Mohr’s Salt Against KMnO4 (Image- Byju’s)
Acid Base Titration with example Mohr’s Salt Against KMnO4 is performed as follows:
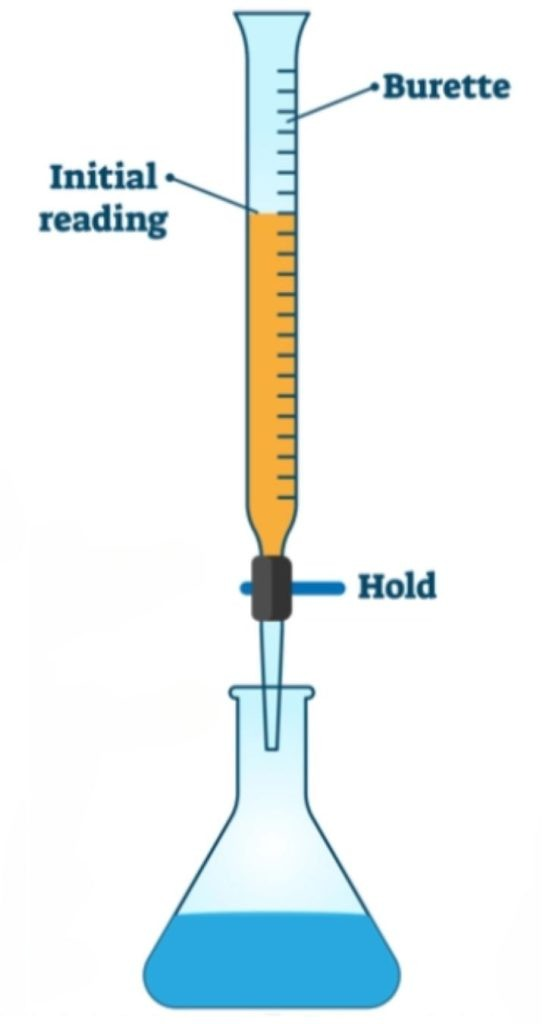
- Wash the burette with distilled water, then KMnO4.
- Fill the burette with KMnO₄ using a funnel up to the zero mark at the top. Do this while the burette is on the ground, as filling it while placed on a table can be difficult—lab benches are high, and many students take practical exams in schools other than their own. This might mean performing the experiment on unfamiliar lab benches.
- Always remember to remove air from the nozzle by opening the stopcock slightly. This ensures accuracy. Dispense the excess KMnO₄ into a separate test tube to prevent spills on the table. The nozzle tip holds some volume. The total volume is measured from the tip to the zero mark at the top of the burette.
- Fill the burette with excess KMnO₄ initially and drain it until the level reaches the zero mark. Note the upper meniscus for these experiments.
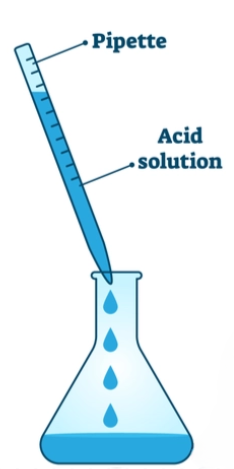
- Use a glass pipette to transfer Mohr’s salt. Partially block the pipette’s opening with your finger and gently inhale to draw the solution into the pipette. This helps pull in enough solution. The ring marked at the top of the stem indicates the 20 mL marking for this 20 mL glass pipette.
- After filling the pipette, keep your finger in place and release it when ready to dispense the Mohr’s salt solution into the conical flask. Ensure the flask is washed with soap and clean water and dried completely to prevent interference with reaction volumes.
- Each drop must be dispensed into the flask, as even a single drop remaining in the pipette could affect your final reading. If you want to avoid using a mouth pipette, you can also use a 20 mL test tube to transfer the Mohr’s salt solution.
- Open the burette stopcock slowly and dispense the KMnO₄ drop by drop. Maintain your eye level parallel to the markings on the burette to avoid parallax error. Keep rotating the conical flask in a circular motion to ensure proper mixing.
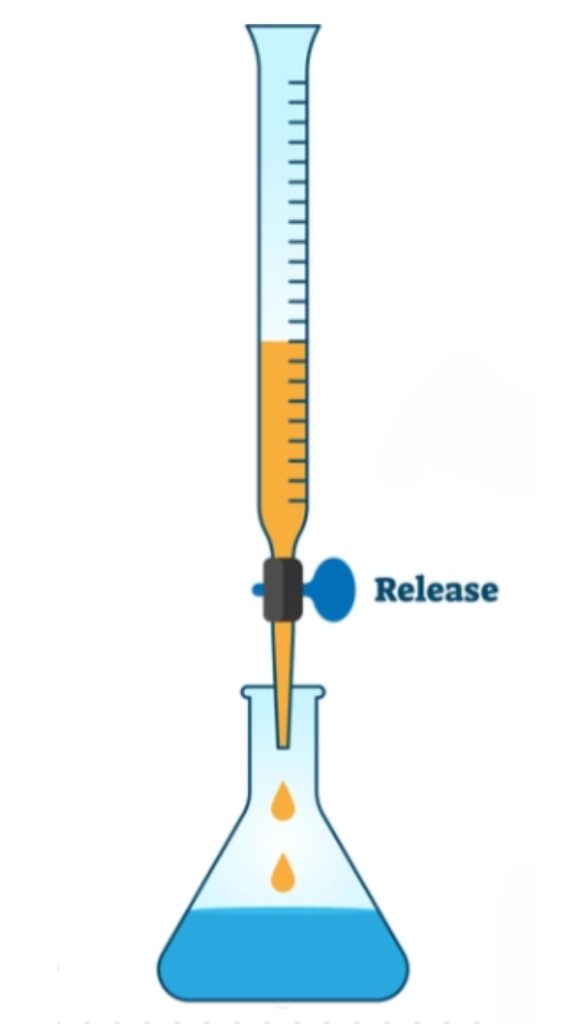
- Approximately 17–20 mL of KMnO₄ is usually required. Carefully monitor the measurement once the burette crosses the 17 mL mark. At this point, control the stopcock with one hand while continuing to rotate the conical flask with the other.
- Take three readings to find the final average value, as this provides a more accurate result. This is also required as per Board Exam rules.
- The endpoint is when a light pink color first appears, not when the solution becomes dark pink or violet. Stop the burette stopcock immediately as soon as the solution turns light pink.
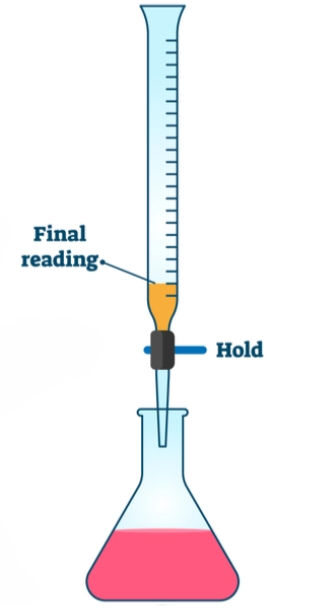
- The moment the solution turns light pink, take the reading from the burette. This reading indicates the amount of KMnO₄ required to neutralize the Mohr’s salt solution.
Titration of Mohr’s Salt with KMnO4: Calculation
Normality of KMnO4 solution:
Consider “y” ml of given KMnO4 solution is equivalent to 20 ml of N/10 Mohr’s salt solution.
According to law of equivalents,
N1V1 = N2V2
N1, N2 are normality of Mohr’s salt and KMnO4 solution respectively.
V1, V2 are volumes of Mohr’s salt and KMnO4 respectively.
1/10 x 20 = N2 x y
N2 = 2/y
N = Normality of given KMnO4 solution = 2/y
Strength of KMnO4 solution:
Strength = Normality x Equivalent mass
Equivalent mass of KMnO4 =
= 158/5 = 31.6 = 2/y x 31.6 g/liter
Molarity of KMnO4 solution:
N = M x Number of electron gained
N = M x 5
M = N/5 moles/ liter
The strength of and molarity of a given KMnO4 solution is found out as 2/y x 31.6 g/l and N/5 moles/liter, respectively.
Results and Discussion
Molarity of given KMnO4 solution is _________ moles/liter
The strength of given potassium permanganate solution is _______ g/L
Precautions
Beware of these traps if you don’t want to feel like this student during your practical exam.
Acid Base Titration with example Mohr’s Salt Against KMnO4 must be done with these secret tips in mind. This will give you an edge over other candidates.
- Don’t dispense a large amount of KMnO₄ at once initially, as this can lead to errors. Even if you have an estimated value in mind, other issues may arise, and the reaction will take longer to complete.
- Keep rotating the conical flask continuously—do not stop even for a second. If you stop by mistake, the KMnO₄ will not mix properly and will remain in the solution. Later, when it dissolves, the color will appear much darker than needed, meaning the endpoint has been crossed. To prevent this, ensure you keep stirring and stop precisely at the point where the solution turns light pink. A single extra drop can result in a deeper pink color, making your reading inaccurate.
Beware of Mechanical Errors during Titration
- Never attempt to add excess KMnO₄ to the burette if you miss the correct endpoint. If you exceed the endpoint and try to adjust by adding more KMnO₄ to the burette to match the expected reading (you know what I mean), it will be a serious mistake. Not only is this considered cheating, which may lead to penalization, but it could also irritate the examiner.
- When you present your result to the examiner, they will check both the burette reading and the color of the solution in the conical flask. If the solution appears darker than the expected light pink, they will know the reading has been manipulated. The correct amount of KMnO₄ cannot produce a darker solution if the concentrations of KMnO₄ and Mohr’s salt are accurate.
- If you make a mistake, repeat the entire experiment. Generally, board practical exams provide ample time to perform three repetitions, with extra time available if needed. Use this opportunity to carefully repeat the experiment and ensure that this time, you stop precisely when the solution reaches the endpoint and turns light pink.
The Acid Base Titration with example Mohr’s Salt against KMnO4 is a common experiment often asked in CBSE, ICSE and State board practical exams.
Frequently Asked Questions (FAQ)/ VIVA
- What is titration?
Titration is a laboratory technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. It involves the gradual addition of a known solution to an unknown solution until the reaction reaches its endpoint.
- What is the endpoint in titration?
The endpoint is the point at which the reaction is complete. It is usually indicated by a color change in the solution when using an indicator or when a stable reading is observed in instrumental titrations.
3. Why should titrations be performed drop by drop near the endpoint?
Adding too much titrant at once can overshoot the endpoint, leading to inaccurate results. Dispensing drop by drop ensures precise observation of the color change and prevents errors.
4. Why do we remove air bubbles from the burette before titration?
Air bubbles in the burette nozzle can cause inaccurate readings, as they can lead to an incorrect volume of titrant being dispensed. Removing air ensures precise measurements.
5. What is a standard solution in titration?
A standard solution is a solution with a precisely known concentration. It is used as the titrant to determine the unknown concentration of another solution.
6. Why is sulfuric acid added in the titration of Mohr’s salt with KMnO₄?
Sulfuric acid provides an acidic medium, which is necessary for the redox reaction between KMnO₄ and Mohr’s salt to occur correctly. Without it, the reaction might not proceed efficiently.












Leave a Reply